Biosimilarity FDA perspective バイオシミラー
(税込) 送料込み
商品の説明
定価 約27,、現在アマゾンの最安値が約17,です。
バイオシミラー薬・FDAについての洋書のハードカバー本です。
●状態:表紙の角がやや擦れていますが、中身は一部のみ読んだほぼ新品です。
●ヤケ:なし
●折り目:なし
●書き込み:なし
●その他、注意事項:
中古品ということをご理解の上ご検討ください。宜しくお願いします商品の情報
| カテゴリー | 本・音楽・ゲーム > 本 > 洋書 |
|---|---|
| 商品の状態 | 未使用に近い |

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

Biosimilarity: The FDA Perspective

Biosimilarity: The FDA Perspective See more 1st Edition1st Edition

An FDA perspective on the assessment of proposed biosimilar

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

The Top 5 Biosimilar Articles for the Week of May 15

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書
The Journey from Biologic to Biosimilar—A Clinical Perspective - ACRP
Biologics | Free Full-Text | The Coming of Age of Biosimilars: A

Pharmacodynamic Biomarkers: Their Role in Biosimilar Product

Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An
休日 Biosimilarity FDA perspective バイオシミラー savingssafari.com

Biosimilar Clinical Trials and US FDA Guidance by ProRelix Info
The Biosimilar Market: What You Need to Know - Retina Today
宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

Biosimilar Clinical Trials and US FDA Guidance | ProRelix Research
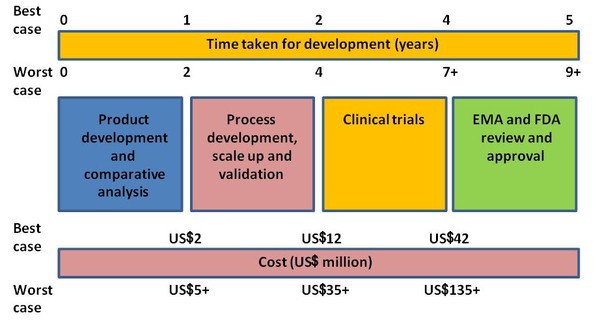
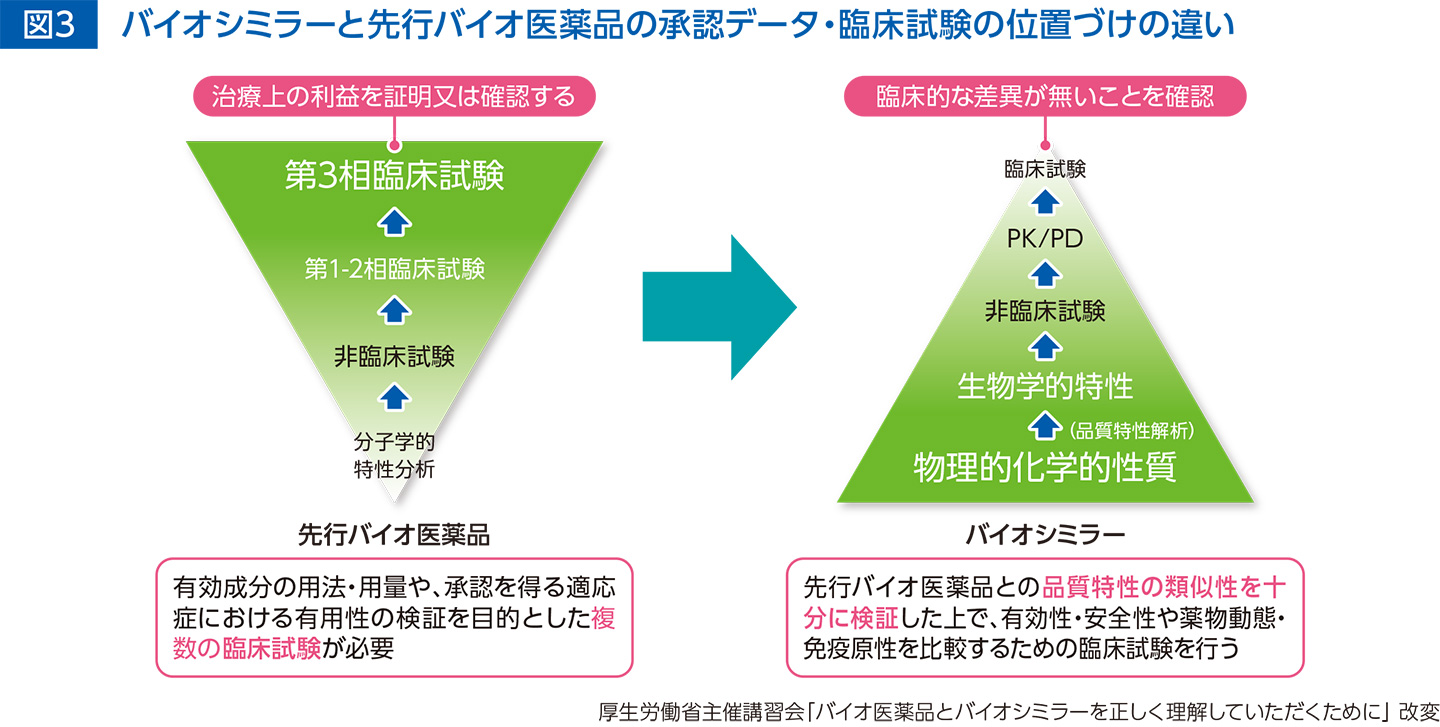
Development of biosimilars

Biosimilar Clinical Trials and US FDA Guidance by ProRelix Info
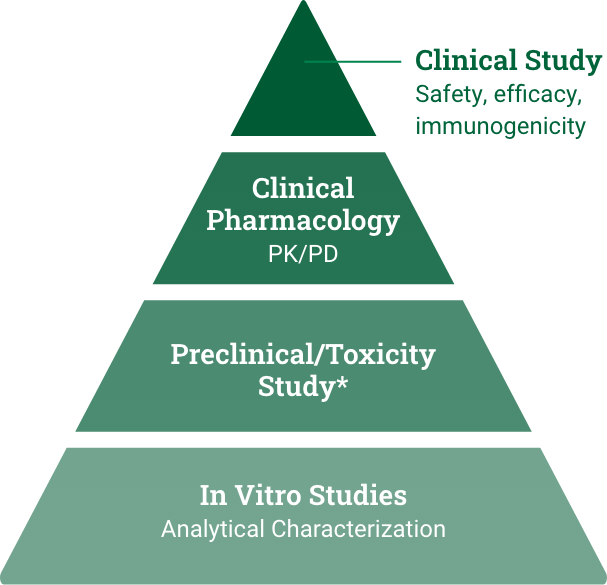
CIMERLI™ | Biosimilar Promise can Expand Patient Access to Cost

Provider and Patient Knowledge Gaps on Biosimilars: Insights From

The Basics of Biosimilars

FDA Releases Biosimilars Guidelines to Little Fanfare

Novartis, Roche, and Pfizer respond to FDA Biosimilar Action Plan

Safety, Immunogenicity and Interchangeability of Biosimilar
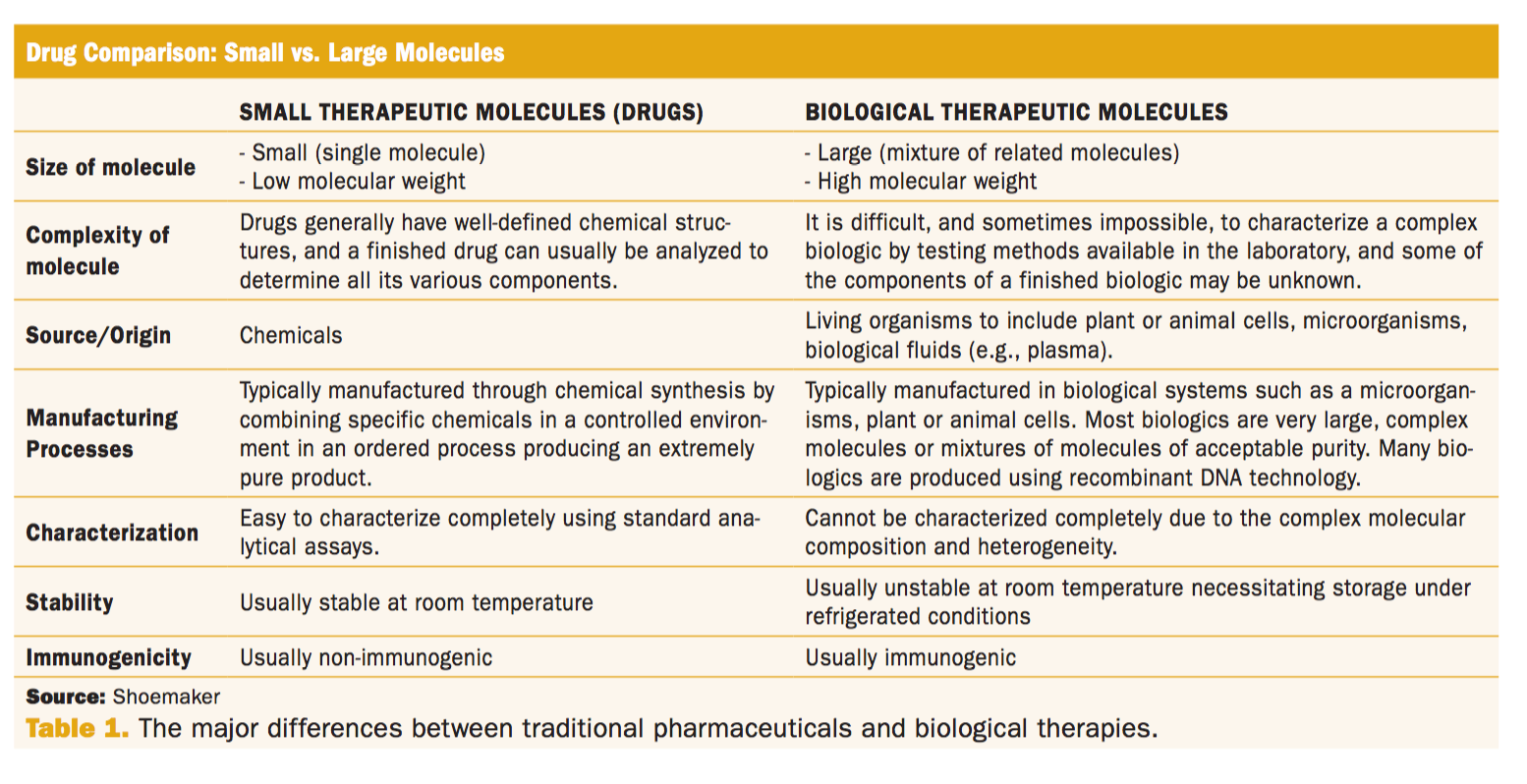
The U.S. Biosimilar Pathway: Policy Precedes Science

Tentative Biosimilar Approvals A Consideration for the US FDA
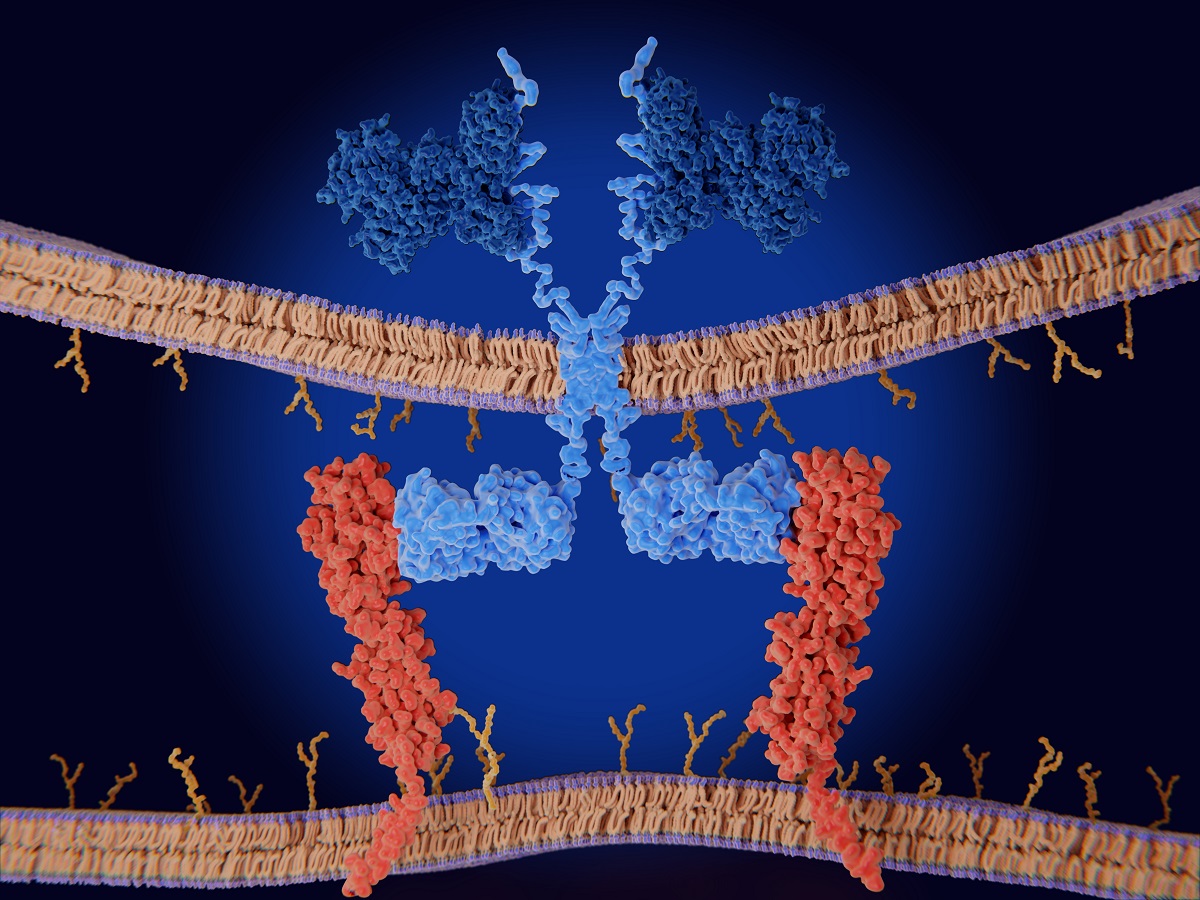
Functional Biosimilarity to Replace Clinical Efficacy Testing of

Biosimilars in Developed Economies: Overview, Status, and
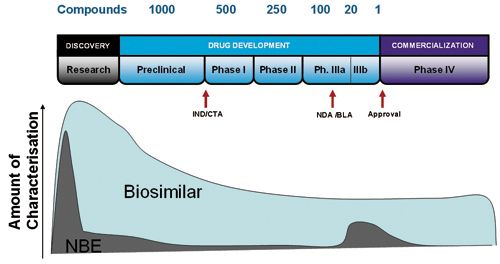
Key Considerations in Biosimilars Development
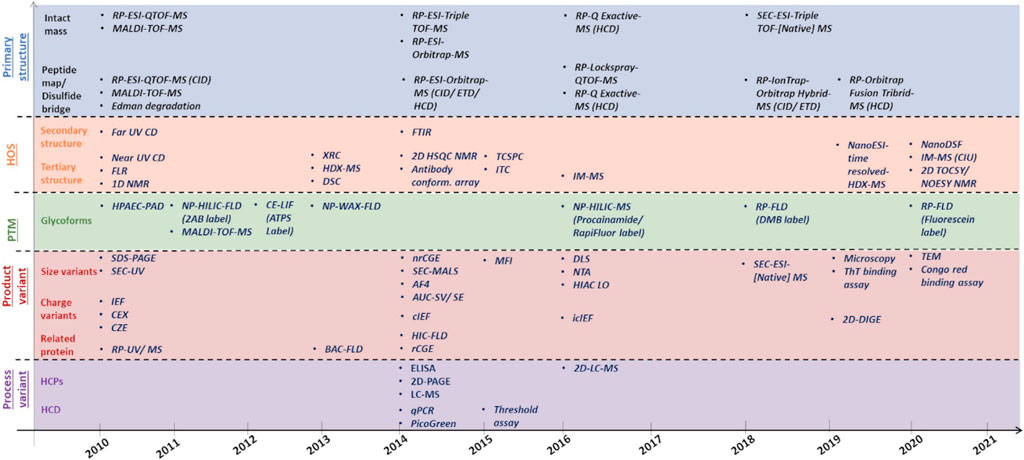
Frontiers | Analytical Similarity Assessment of Biosimilars

Unpacking an NEJM Perspective on the Lackluster Biosimilar Uptake

FDA Perspectives on Biosimilar BLA-Manufacturing (28of33) Quality – Oct. 16-17, 2019
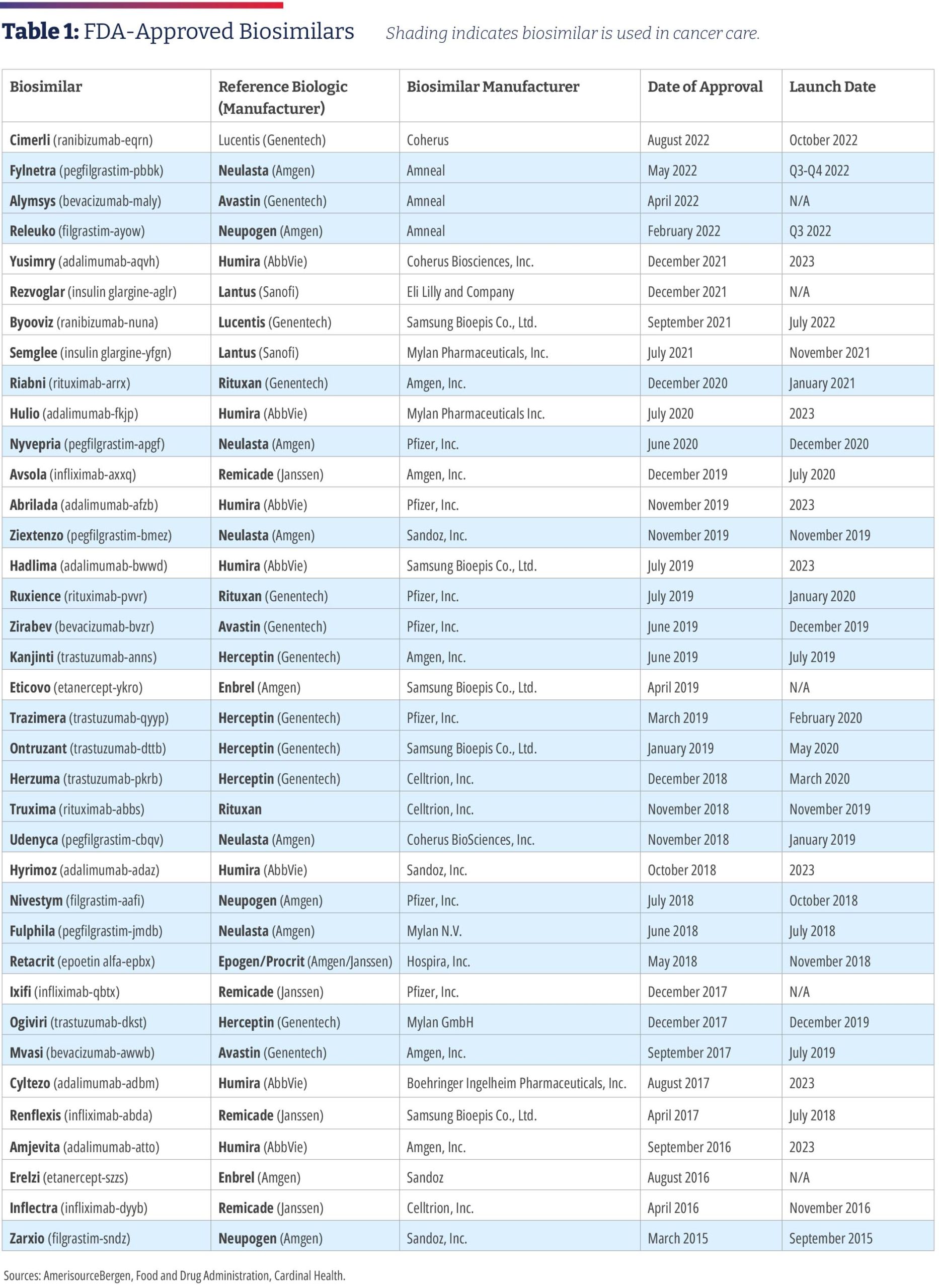
The Promise Of Biosimilars In Cancer Care And Reality Of The U.S.

FDA approval of biosimilars in the United States | Download Table
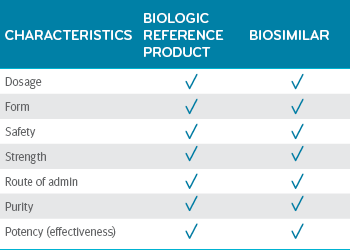
Biosimilars are here to help, really - Prime Therapeutics LLC

Biosimilars

Biosimilar or Not: Physicochemical and Biological Characterization
Biosimilars 2022 Year in Review

From our perspective: Biosimilar product labeling | FDA

The Rise of Biosimilars: Success of the BPCIA? (Part I) - Bill of


商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています














